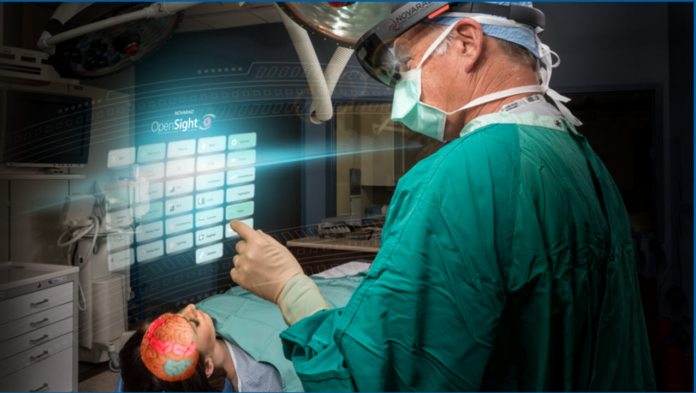
OpenSight is used to overlay 3D graphics on patients to help with things like surgical planning whilst also maintaining awareness of their surroundings. For example, a surgeon can mark an appropriate entry point for a needle, or it can be used to aid trainees. As far as we know, this is the first HoloLens app to receive 510(k) clearance. The software supports use my multiple headsets at once, with a specific training version with accounts for use on cadavers. “This is transformative technology that will unite preoperative imaging with augmented reality to improve the precision, speed and safety of medical procedures,” said Dr. Wendell Gibby, Novarad CEO and co-creator of OpenSight. “This internal visualization can now be achieved without the surgeon ever making an incision, improving outcomes in a world of more precise medicine.”
FDA Limitations
The decision for FDA approval was made on September 18, 2018, but was only widely reported recently.